Diabetic retinopathy اعتلال الشبكية السكري
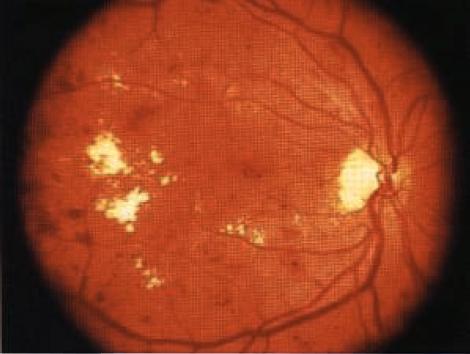
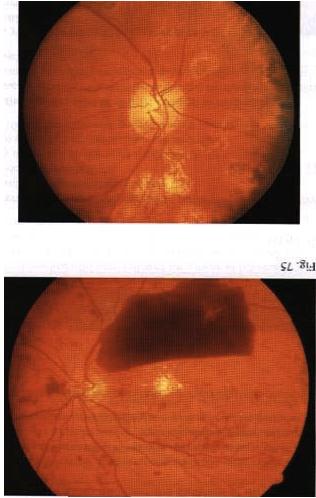
INSTRUCTION Examine the fundus in these patients. SALIENT FEATURES You will be expected to comment on whether there is background or proliferative retinopathy. You may have a clue about the underlying diabetes, either from a diabetic chart or from the presence of diabetic fruit juices at the bedside. History · Gradual or acute loss of vision. · History of floaters. · History of diabetes, hypertension. · Ask about renal disease (renal-retinal syndrome of diabetes). Examination Patient I has background retinopathy (Fig. 74), caused by microvascular leakage into the retina. Microaneurysms. usually seen in the posterior pole temporal to the fovea. · Dot and blot haemorrhages. · Hard exudates. · Cottonwool spots. DIAGNOSIS This patient has dot and blot haemorrhages and cottonwool spots (lesions), probably due to diabetic retinopathy (aetiology), and has good visual acuity (lunctional status). QUESTIONS What symptoms will this patient have? The patient will be asymptomatic as the macula is spared. How would you manage such a patient? · Treat diabetes and associated hypertension. · Annual fundal examination. The Early Treatment of Diabetic Retinopathy Study (ETDRS) has established that early peripheral (panretinal) argon laser photocoagulation is not indicated for mild to moderate non-proliferative retinopathy. Early treatment in the form of argon laser photocoagulation applied directly to leaking microaneurysms, as well as grid photo-coagulation applied to diffuse areas of leakage and thickening, is highly beneficial. SALIENT FEATURES Patient 2 has diabetic maculopathy, caused by oedema and/or hard exudates. · Signs of background retinopathy with hard exudates or oedema of the macula. QUESTIONS What symptoms may this patient have? There will be a gradual impairment of central vision, such as difficulty in reading small print or seeing road signs. ADVANCED-LEVEL QUESTIONS What are the signs of macular oedema? Macular oedema includes any of the following signs: · Retinal thickening at or within 500 pm of the centre of the macula. · Retinal thickening of one disc area or larger, in any part of the retina which is within the one-disc diameter from the centre of the macula. · Hard exudates at or within 500 [tm of the centre of the macula. How will you manage this patient? Non-urgent referral to an ophthalmologist for photocoagulation which will stabilize (seldom improve) visual acuity in 50% of patients. The ability to alter the course of visual loss in diabetic macular oedema favourably is a major advance but patients must be cautioned that the most likely result of treatment is stabilization, not im-provement, of visual acuity. What is the principal mechanism of visual loss in non-proliferative retinopathy? The principal mechanisln of visual h)ss in non-proliferative retinopathy is macular oedema, which results from focal vascular leakage from microaneurysms in the macular capillaries, as well as diffuse vascular leakage. With time, areas of leakage progress to macular thickening associated with hard exudates or cystoid changes. What are the different types of clinical presentation ? Patients may present with no visual symptoms, paracentral scotomata or various degrees of central visual loss. Consequently, the diagnosis and management of macular oedema depend crucially on the determination of macular thickening by fundus examination. Ophthalmoscopy detects intraretinal haemorrhages and hard exudates but does not detect substantial retinal thickening. A critical evaluation of retinal thickening requires stereoscopic examination of the retina by slit-lamp bio-microscopy with lens for retinal visualization or stereoscopic fundus photography. SALIENT FEATURES Patient 3 has preproliferative retinopathy which is uncommon and is caused by retinal hypoxia. · Cottonwool spots. · Venous dilatation, beading, looping or sausage-like segmentation. · Arteriolar narrowing. · Haemorrhages - large dark blots. · Intraretinal microvascular abnormalities. QUESTIONS What symptoms may this patient have? The patient is asymptomatic if the macula is spared. How would you manage this patient? Semiurgent referral to the ophthalmologist for close follow-up to enable early detection and treatment of proliferative retinopathy. SALIENT FEATURES Patient 4 has proliferative retinopathy caused by retinal hypoxia, usually seen in insulin-dependent diabetic retinopathy (Figs 75 and 76). · Neovascularization around the disc (NVD) or away from the disc - in early stages the vessels are bare and flat and easily missed. In later stages they are elevated and may be associated with a white fibrous component. · Presence of laser burns (in treated cases). QUESTIONS What symptoms may this patient have? The patient is asymptomatic in the absence of complications. How would you manage this patient? Urgent referral to an ophthalmologist for laser treatment. What are the complications of proliferative retinopathy? · Vitreous haemorrhage. · Traction retinal detachment. · Rubeosis irides. · Rubeotic glaucoma - some of these patients can experience partial restoration of vision by microsurgery called pars plana vitrectomy. ADVANCED-LEVEL QUESTIONS What is the prevalence of retinopathy in diabetes? The overall prevalence is about 25%. It is 40% in insulin-dependent diabetes mellitus (IDDM) and 20% in non-insulin-dependent diabetes mellitus (NIDDM). What is the relationship between the duration of diabetes and retinopathy? There is a close relationship: in patients diagnosed as having diabetes before the age of 30 years, the incidence of retinopathy is about 50% after 10 years and 90% after 30 years. It is unusual for retinopathy to develop within 5 years of onset of diabetes; however, 5% of patients with NIDDM have background retinopathy at presentation. What associated systemic conditions worsen diabetic retinopathy? · Pregnancy. · Hypertension. · Anaemia. · Renal failure. What is the relationship between diabetic control and retinopathy? The Diabetes Control and Complications Trial compared intensive treatment for blood glucose control with conventional treatment in patients with and without retinopathy at baseline who were followed for a mean of 6.5 years. Intensive treat-ment has profound benefits in both subgroups and was associated with a reduction in the incidence of both the development of new retinopathy and the risk of pro-gression of existing retinopathy. How often would you screen diabetic patients for retinopathy? · Non-insulin-dependent diabetics: annually. · Insulin-dependent diabetics: - Newly diagnosed: no screening for the first 5 years. -Five to ten years from initial diagnosis: annually. Over 10 years after initial diagnosis: every 6 months. What is the earliest sign of retinal change in diabetes? An increase in capillary permeability, evidenced by the leakage of dye into the vitreous humour after fluorescein injection, is the earliest sign of retinal change in diabetes mellitus. What do you know about the pathogenesis of retinal new vessel formation ? It is not completely understood and current theories emphasize the production of angiogenic factors by areas of ischaemic and hypoxic retina. More recently, vas-cular endothelial growth factor (VEGF) has been isolated from ocular fluid and is an endothelial cell-specific angiogenic factor whose production is increased by hypoxia: it has been implicated in the neovascularization seen in diabetic retino-pathy and retinal vein occlusion. What do you know about photocoagulation? Photocoagulation is a technique whereby several thousand lesions are produced over a 2-week therapy period with lasers. Panretinal photocoagulation reduces the risk of severe visual loss. The laser is used to ablate a portion of the retina and does not directly cauterize the neovascularization. It is believed that the regression of neovascularization due to laser is a result of the destruction of ischaemic and hypoxic retina with reduction in angiogenic factors. Photocoagulation decreases the incidence of haemorrhage or scarring in proliferative retinopathy. Photocoagulation is also useful in the treatment of microaneurysms, haemor-rhages and oedema. Some loss of peripheral vision may be inevitable with this technique. What surgical technique may be used for a non-resolving vitreous haemorrhage and retinal detachment? Pars plana vitrectomy may be used hut is often complicated by retinal tears, retinal detachment, glaucoma, infection, cataracts and loss of the eye.